

Explain the reason for this type of colour change.Īnswer: Alkali metals dissolve in liquid ammonia and give deep blue solutions which are conducting in nature because ammoniated electrons (e –(NH 3) y) absorb energy in the visible region of light and impart blue colour. When alkali metal dissolves in liquid ammonia, the solution can acquire different colours. Due to this, K and Cs are used in photoelectric cells rather than lithium.ġ0. As a result, these metals easily emit electrons on exposure to light. Why are potassium and caesium, rather than lithium used in photoelectric cells?Īnswer: Potassium and caesium have much lower ionization enthalpy than that of lithium. That is why these metals are not obtained by chemical reduction methods.ĩ. Explain why can alkali and alkaline earth metals not be obtained by chemical reduction method.Īnswer: Alkali and alkaline earth metals are themselves better reducing agents, and reducing agents better than alkali metals are not available. (c) Both the elements have the tendency to form covalent compounds.Ĩ. (b) Both react with O 2 to form monoxides. (a) Both react with nitrogen to form nitrides. In what ways lithium shows similarities to magnesium in its chemical behaviour?Īnswer: The similarities of lithium with magnesium are: Alkali metals due to lower ionization enthalpy are more electropositive than the corresponding group 2 elements.ħ. (iii) Solubility of hydroxides of alkali metals are higher than that of alkaline earth metals. (ii) Basicity of oxides of alkali metals are higher than that of alkaline earth metals. Compare the alkali metals and alkaline earth metals with respect to (i) ionization enthalpy, (ii) basicity of oxides, (iii) solubility of hydroxides.Īnswer: (i) Because of smaller atomic size the ionization enthalpy of alkaline earth metals are higher than those of the corresponding alkali metals.
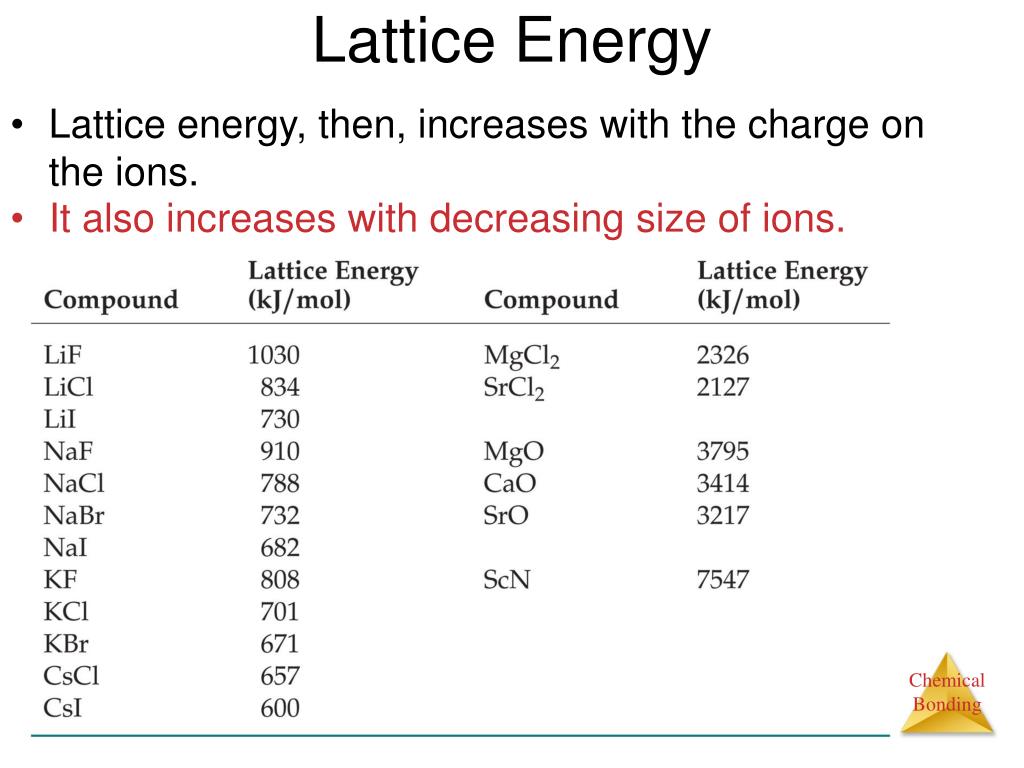
The atomic radius of potassium is greater than sodium, so the ionisation energy of potassium is less than sodium, which makes sodium less reactive than potassium.Ħ. We know that the atomic radius increases down the group and ionisation energy is inversely proportional to the atomic radius. Since Ionization enthalpy of potassium is less than that of sodium, potassium is more reactive than sodium. Explain why is sodium less reactive than potassium.Īnswer: Ionization enthalpy of potassium is 419 kJ mol -1 and Ionization enthalpy of sodium is 496 KJ mol -1. So, the oxidation state of Na in Na 2O 2 is +1.Ĭheck out: 80 Conceptual Questions of the P-Block Elements Class 12ĥ. Oxidation state of O is -1 as it is peroxide bond. Find out the oxidation state of sodium in Na 2O 2.Īnswer: Let x be the oxidation state of Na in Na 2O 2 That’s why they always exist in combined state in nature.Ĥ. Why are alkali metals not found in nature?Īnswer: Alkali metals are highly reactive in nature. (e) Electropositive character increases on going down the group.ģ. (d) They are less electropositive than alkali metals.

(b) Ionisation energy goes on decreasing down the group. (a) Atomic size goes on increasing down the group. Discuss the general characteristics and gradation in properties of alkaline earth metals.Īnswer: The general characteristics and gradation in properties of alkaline earth metals are: (c) Alkali metals dissolve in liquid ammonia to form blue and conducting solution.Ģ. Exploring 74 Important Trends in S and P Block Elements


 0 kommentar(er)
0 kommentar(er)
